The future of RNA is personalized
At Nutcracker Therapeutics, everything we create and do is purpose-built for RNA. We offer precision and right-sized solutions that scale with drug development, whether your therapeutic is for general use or personalized. Our CRDMO services are designed to support the broad potential of RNA-based drugs throughout the full development lifecycle.
Our CRDMO workflows and capabilities harness the power of the Nutcracker® Manufacturing Unit’s (NMU’s) microfluidics and biochip-based automation, scaling to meet any needs throughout your development journey.
RNA therapeutic platform
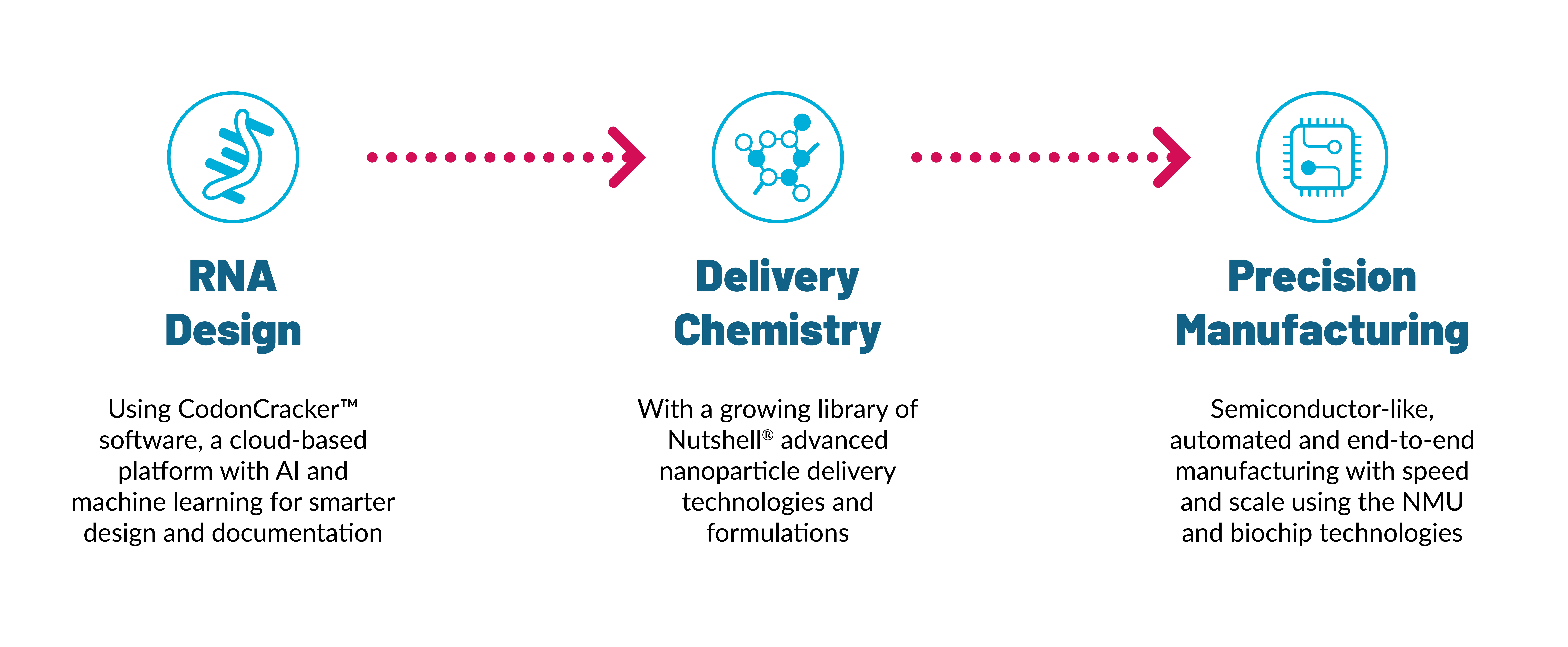
On-demand capacity that scales when and how you need it
The NMU is a microfluidics instrument that utilizes single-use, semiconductor-like biochips with on-chip QA/QC measurements to consistently deliver best-in-class, reproducible RNA, that can accommodate a diverse range of workflows, including a wide array of nanoparticles and RNA types.
Combining the biochips and the NMU allows the compression of the entire manufacturing process into a printer-sized, self-contained instrument that enables a scale-out strategy for rapid increase of output — from small scale manufacturing of personalized cancer therapeutics to larger scale manufacturing of non-personalized RNA medicines. This strategy replicates the NMU at every scale in a modular fashion, without the need for process changes.
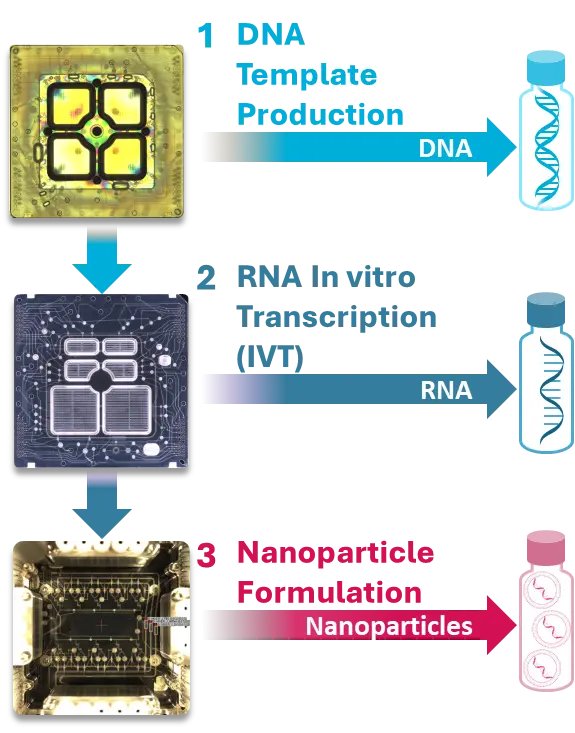
Nutcracker Therapeutics’ RNA manufacturing facility and expertise provide consistent quality and reproducibility from discovery to late-stage clinical trials. Its 8,000 ft² GMP facility supports an annual manufacturing capacity for 2,000 personalized medicines that’s automated and end-to-end. Our NMU-based small footprint and single-use components enable rapid parallel processing of multiple medicines.
The combination of the NMU and biochip technologies address the unique challenges of RNA manufacturing, offering the industry’s lowest cost per individualized therapy while maintaining superior performance and production control to minimize reagent waste.
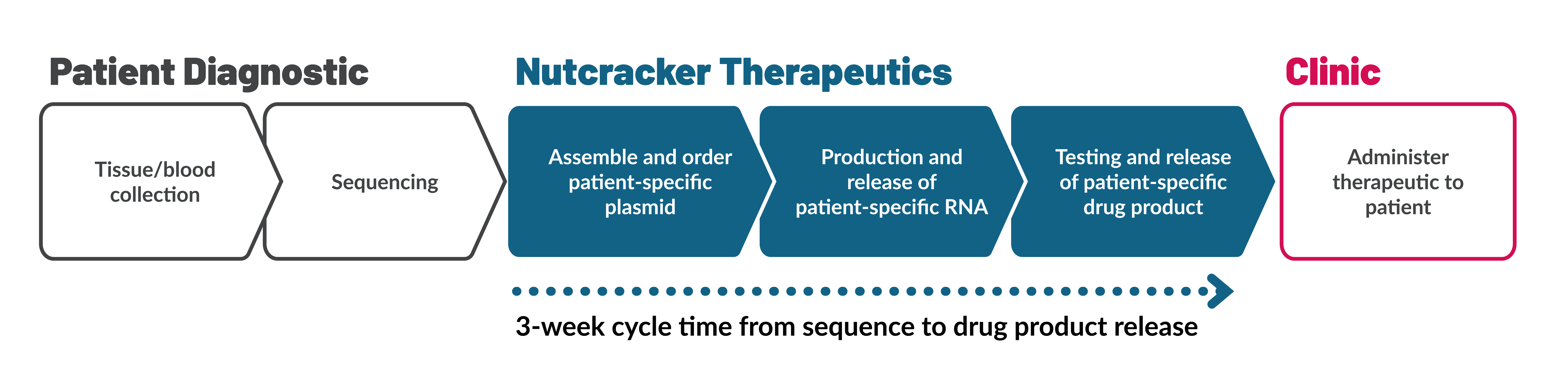
Integrated facility workflow enables rapid cycle time from sequence to patient delivery for individualized therapies. A 3-week process is critical in oncology, across clinical settings, in early stage and advanced disease. Speed of processing is also important in other therapeutic areas such as pandemic preparedness.
Accelerate Your RNA Therapeutic Development
Leverage our expertise and the NMU to advance your RNA therapeutic with unparalleled precision, efficiency, cost and speed.
Contact us today to explore how we can support your drug development.

